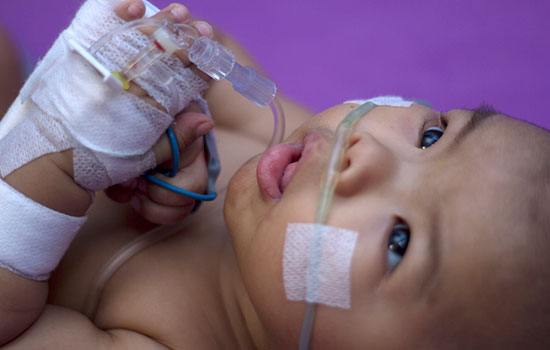
Respiratory Syncytial Virus (RSV) has long been a cause of concern for parents and healthcare providers. However, a beacon of hope has emerged in the form of a new RSV vaccine designed specifically for infants. On August 3, the Centers for Disease Control and Prevention (CDC) announced it is recommending a new immunization starting this fall to help protect all infants under 8 months old and some older babies at risk of contracting an RSV-related illness
The Need for Action
RSV is one of the most common causes of childhood respiratory infections in infants, often leading to hospitalizations. According to the CDC, in the US an estimated 58,000 to 80,000 children under the age of 5, mostly infants, are hospitalized each year due to RSV with some requiring oxygen, intravenous (IV) fluids or mechanical ventilation. Approximately 100 to 300 of those under 5 years of age die due to RSV. According to the World Health Organization (WHO), RSV accounts for an estimated 33.1 million cases of acute lower respiratory tract infections in children under 5 each year, resulting in approximately 59,600 deaths worldwide.
About the New Vaccine
Recent clinical trials of the new vaccine, Nirsevimab, have shown promising results. A study published in the New England Journal of Medicine stated the vaccine demonstrated an efficacy rate of over 80% in preventing severe RSV in lower respiratory tract infections in infants. The vaccine was approved by the U.S. Food and Drug Administration (FDA) last month and is administered as an injection and provides infants and toddlers with the antibodies needed to protect them against severe RSV illnesses that can be life-threatening for infants.
Looking Ahead
The introduction of a new RSV vaccine tailored for infants has the potential to significantly reduce the global burden of RSV-related illnesses. In the U.S. the CDC is working to make the vaccine available through the Vaccines for Children Program, which provides recommended vaccines and immunizations at no cost to about half the nation’s children. Healthcare providers and parents alike eagerly await the opportunity to provide enhanced protection to the most vulnerable members of our population.
To learn more about RSV in infants visit the CDC webpage here.
Sources:
Centers for Disease Control and Prevention. “CDC Recommends a Powerful New Tool to Protect Infants from the Leading Cause of Hospitalizations.” Accessed from: https://www.cdc.gov/rsv/high-risk/infants-young-children.html
World Health Organization. “Respiratory syncytial virus infection.” Accessed from https://onlinelibrary.wiley.com/doi/10.1111/irv.12726
U.S. Food and Drug Administration. “FDA Approves New Drug to Prevent RSV in Babies and Toddlers.” Accessed from: https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-prevent-rsv-babies-and-toddlers
Madhi, Shabir A., et al. “Efficacy of the RSV F nanoparticle vaccine candidate in a maternal immunization model.” New England Journal of Medicine 384.5 (2021): 438-449